Mixed Tree Nut Snacks Compared to Refined Carbohydrate Snacks Resulted in Weight Loss and Increased Satiety during Both Weight Loss and Weight Maintenance: A 24-Week Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Endpoints
2.4. Weight and Body Composition Measurement
2.5. Plasma Lipids, Cholesterol, and Inflammatory Markers
2.6. Plasma Fatty Acid Panel
2.7. Statistical Analyses
3. Results
3.1. Participant Flow and Baseline Characteristics
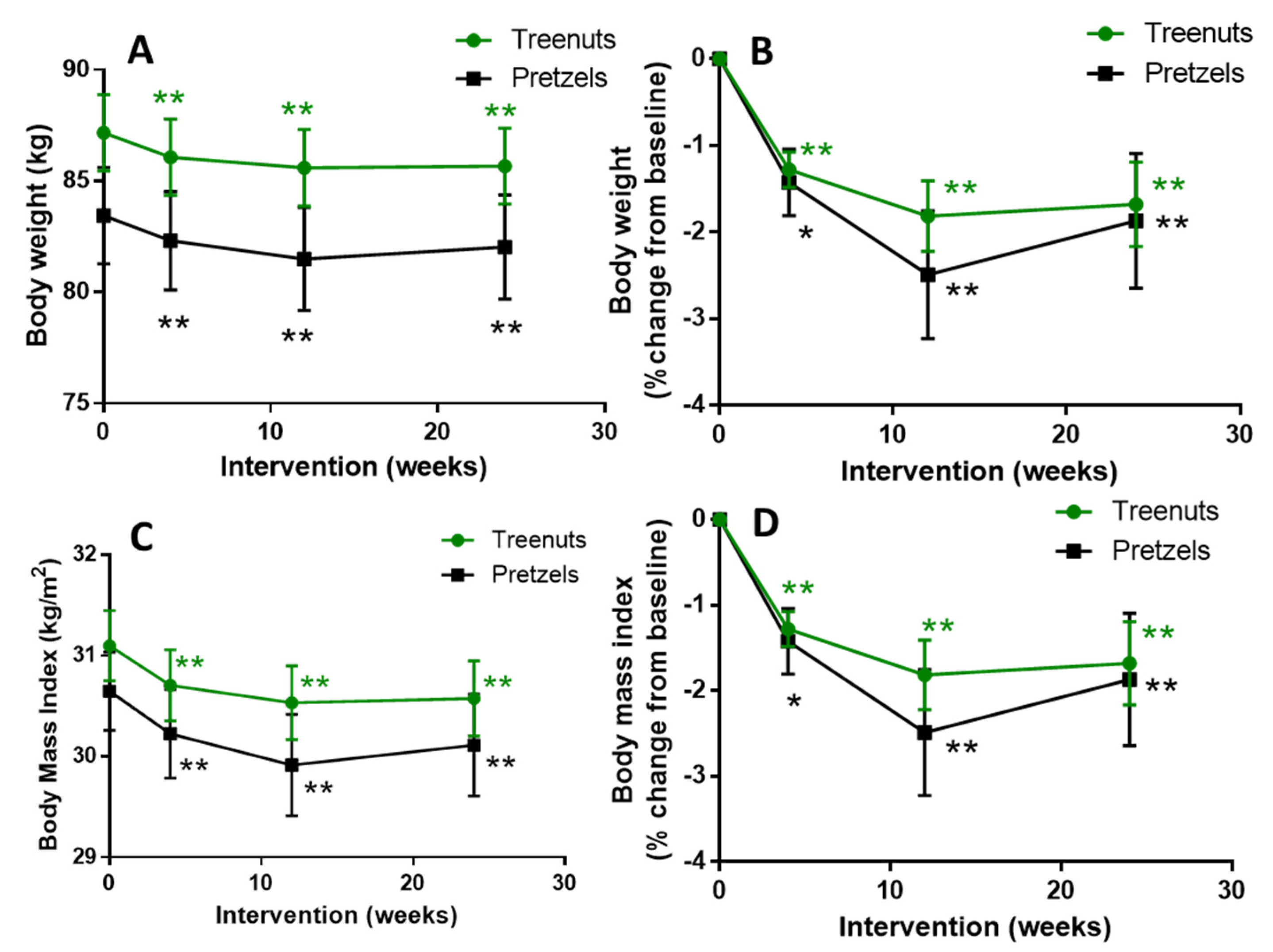
3.2. Compliance, Body Weight, Body Composition, Blood Pressure, and Heart Rate
3.3. Plasma Lipids and Fatty Acid Panel
3.4. Serum Inflammatory Markers
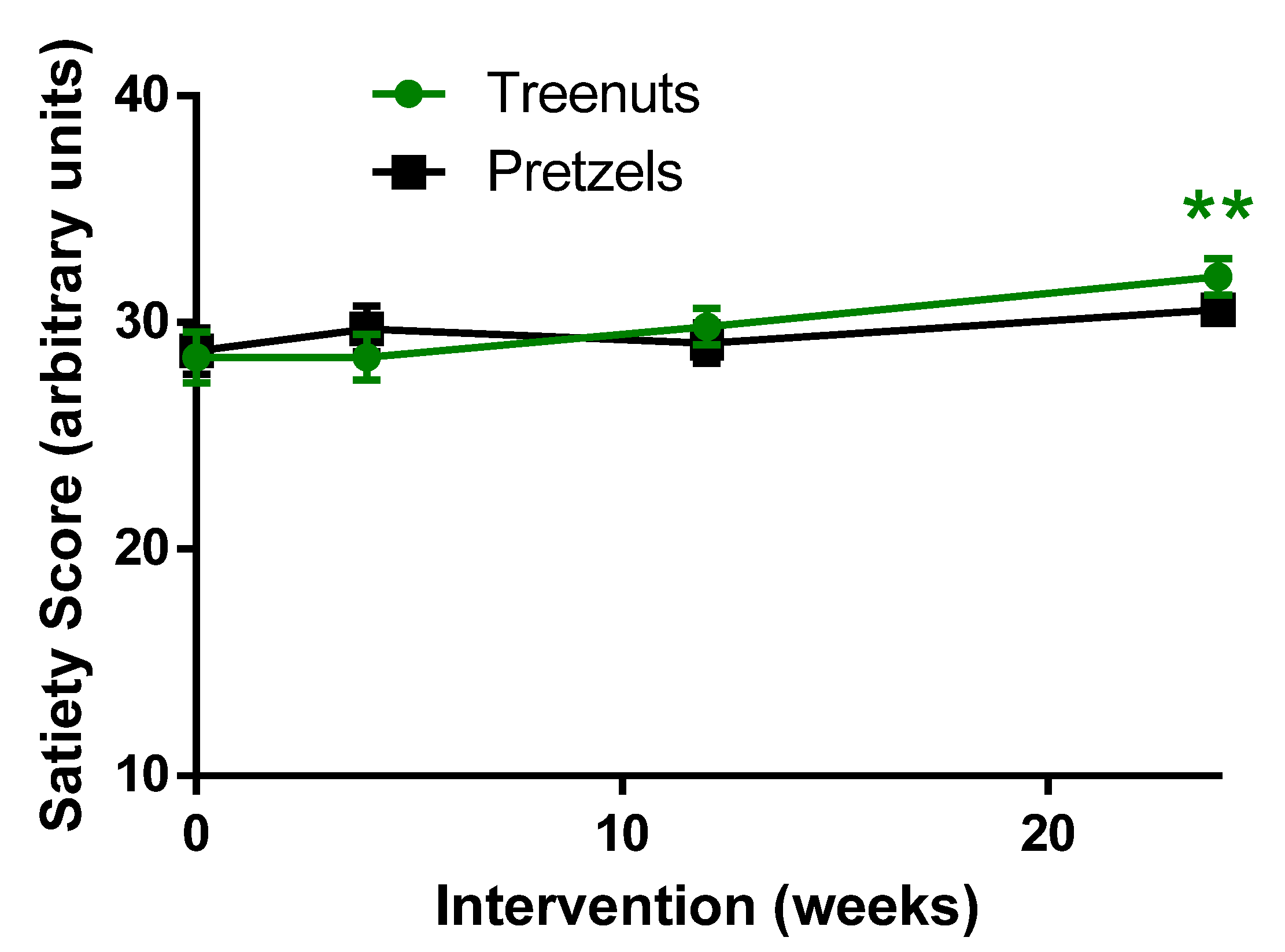
3.5. Satiety Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Reyes, A.; Camara-Martos, F.; Molina-Luque, R.; Romero-Saldana, M.; Molina-Recio, G.; Moreno-Rojas, R. Changes in body composition with a hypocaloric diet combined with sedentary, moderate and high-intense physical activity: A randomized controlled trial. BMC Women’s Health 2019, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Rojas-Rueda, D.; Basora, J.; Ros, E.; Salas-Salvado, J. Nut intake and adiposity: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2013, 97, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Hu, F.B. Long-term associations of nut consumption with body weight and obesity. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 408S–411S. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Nut consumption, weight gain and obesity: Epidemiological evidence. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21 (Suppl. 1), S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Sabate, J.; Beeson, W.L.; Strahan, T.M. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch. Intern. Med. 1992, 152, 1416–1424. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ 1998, 317, 1341–1345. [Google Scholar] [CrossRef]
- Albert, C.M.; Gaziano, J.M.; Willett, W.C.; Manson, J.E. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch. Intern. Med. 2002, 162, 1382–1387. [Google Scholar] [CrossRef]
- Li, Z.; Song, R.; Nguyen, C.; Zerlin, A.; Karp, H.; Naowamondhol, K.; Thames, G.; Gao, K.; Li, L.; Tseng, C.H.; et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J. Am. Coll. Nutr. 2010, 29, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.S.; Shin, E.C.; Shewfelt, R.L.; Eitenmiller, R.R.; Pegg, R.B. Update on the healthful lipid constituents of commercially important tree nuts. J. Agric. Food Chem. 2011, 59, 12083–12092. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Tree Nuts and Peanuts as a Source of Natural Antioxidants in our Daily Diet. Curr. Pharm. Des. 2020, 26, 1898–1916. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Vlase, L.; Popa, D.S. Anti-aging potential of tree nuts with a focus on the phytochemical composition, molecular mechanisms and thermal stability of major bioactive compounds. Food Funct. 2018, 9, 2554–2575. [Google Scholar] [CrossRef] [PubMed]
- Roncero, J.M.; Alvarez-Orti, M.; Pardo-Gimenez, A.; Rabadan, A.; Pardo, J.E. Review about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef]
- Sabate, J.; Cordero-Macintyre, Z.; Siapco, G.; Torabian, S.; Haddad, E. Does regular walnut consumption lead to weight gain? Br. J. Nutr. 2005, 94, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Piernas, C.; Popkin, B.M. Snacking increased among U.S. adults between 1977 and 2006. J. Nutr. 2010, 140, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, C.D.; Moody, R.C.; Shanks, J.; Stock, M.J.; Geissler, C. Sources of energy from meals versus snacks in 220 people in four age groups. Eur. J. Clin. Nutr. 1995, 49, 33–41. [Google Scholar]
- Bagga, D.; Capone, S.; Wang, H.J.; Heber, D.; Lill, M.; Chap, L.; Glaspy, J.A. Dietary modulation of omega-3/omega-6 polyunsaturated fatty acid ratios in patients with breast cancer. J. Natl. Cancer Inst. 1997, 89, 1123–1131. [Google Scholar] [CrossRef][Green Version]
- Rock, C.L.; Flatt, S.W.; Barkai, H.S.; Pakiz, B.; Heath, D.D. Walnut consumption in a weight reduction intervention: Effects on body weight, biological measures, blood pressure and satiety. Nutr. J. 2017, 16, 76. [Google Scholar] [CrossRef]
- Foster, G.D.; Shantz, K.L.; Vander Veur, S.S.; Oliver, T.L.; Lent, M.R.; Virus, A.; Szapary, P.O.; Rader, D.J.; Zemel, B.S.; Gilden-Tsai, A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am. J. Clin. Nutr. 2012, 96, 249–254. [Google Scholar] [CrossRef]
- Abazarfard, Z.; Salehi, M.; Keshavarzi, S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: A randomized controlled clinical trial. J. Res. Med Sci. 2014, 19, 457–464. [Google Scholar]
- Fantino, M.; Bichard, C.; Mistretta, F.; Bellisle, F. Daily consumption of pistachios over 12 weeks improves dietary profile without increasing body weight in healthy women: A randomized controlled intervention. Appetite 2020, 144, 104483. [Google Scholar] [CrossRef] [PubMed]
- Godwin, N.; Roberts, T.; Hooshmand, S.; Kern, M.; Hong, M.Y. Mixed Nuts May Promote Satiety While Maintaining Stable Blood Glucose and Insulin in Healthy, Obese, and Overweight Adults in a Two-Arm Randomized Controlled Trial. J. Med. Food 2019, 22, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Sweeney, L.L.; Liu, X.; Mantzoros, C.S. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity 2010, 18, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Sayer, R.D.; Dhillon, J.; Tamer, G.G.; Cornier, M.A.; Chen, N.; Wright, A.J.; Campbell, W.W.; Mattes, R.D. Consuming Almonds vs. Isoenergetic Baked Food Does Not Differentially Influence Postprandial Appetite or Neural Reward Responses to Visual Food Stimuli. Nutrients 2017, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.Y.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Inclusion of Almonds in a Cholesterol-Lowering Diet Improves Plasma HDL Subspecies and Cholesterol Efflux to Serum in Normal-Weight Individuals with Elevated LDL Cholesterol. J. Nutr. 2017, 147, 1517–1523. [Google Scholar] [CrossRef]
- Dikariyanto, V.; Smith, L.; Francis, L.; Robertson, M.; Kusaslan, E.; O’Callaghan-Latham, M.; Palanche, C.; D’Annibale, M.; Christodoulou, D.; Basty, N.; et al. Snacking on whole almonds for 6 weeks improves endothelial function and lowers LDL cholesterol but does not affect liver fat and other cardiometabolic risk factors in healthy adults: The ATTIS study, a randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 1178–1189. [Google Scholar] [CrossRef]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; O’Brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.; Kendall, C.W.; Gascoyne, A.M.; Bazinet, R.P.; Bashyam, B.; Lapsley, K.G.; Augustin, L.S.; Sievenpiper, J.L.; Jenkins, D.J. Effect of almond consumption on the serum fatty acid profile: A dose-response study. Br. J. Nutr. 2014, 112, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Gayathri, R.; Jaacks, L.M.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew Nut Consumption Increases HDL Cholesterol and Reduces Systolic Blood Pressure in Asian Indians with Type 2 Diabetes: A 12-Week Randomized Controlled Trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef] [PubMed]
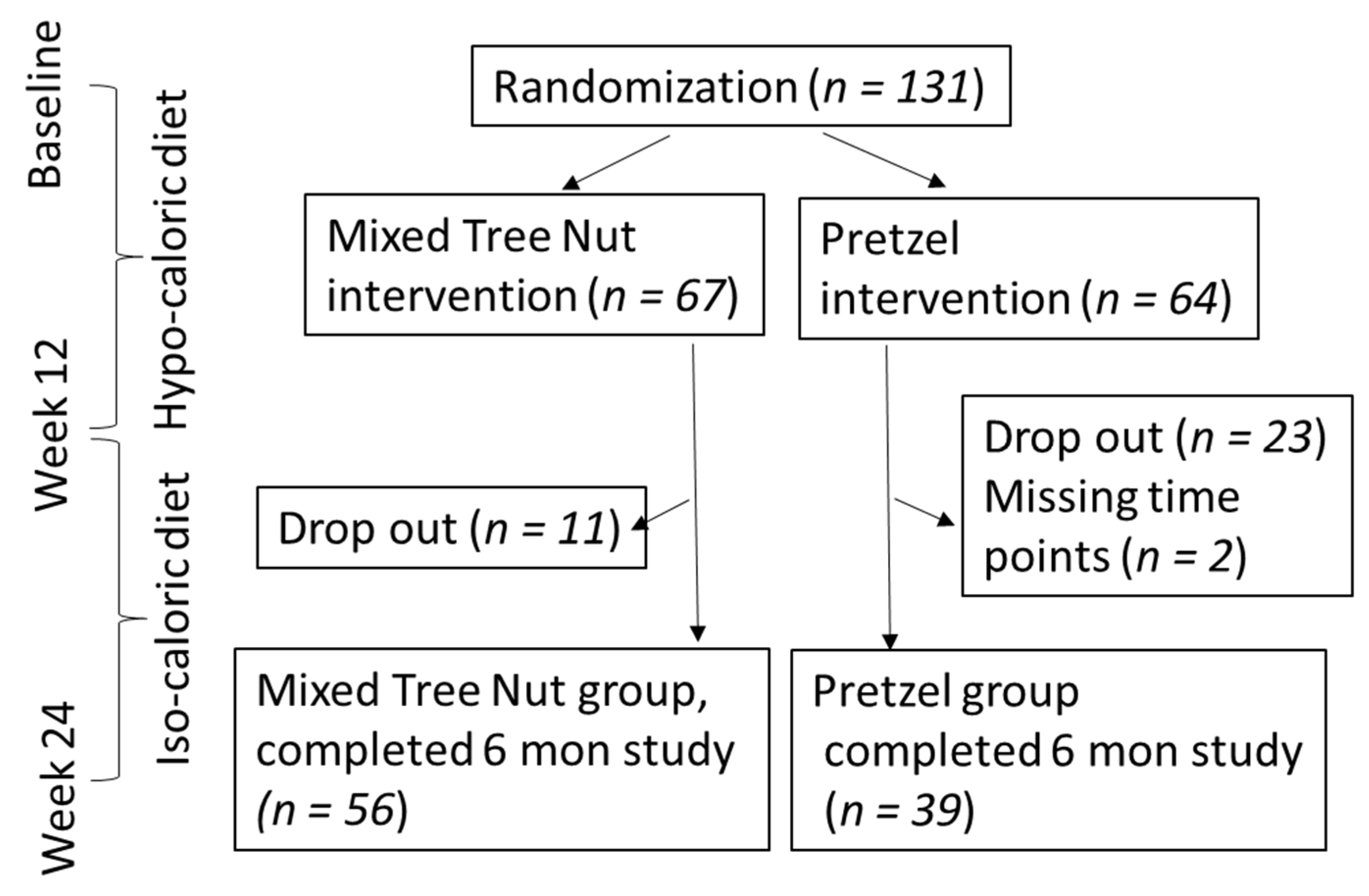

| Tree Nut (N = 56) | Pretzel (N = 39) | p-Value | |
|---|---|---|---|
| Age (years) | 48.3 ± 1.9 | 46.9 ± 1.7 | 0.8115 |
| Gender (F/M) | 39/17 | 32/7 | 0.2313 |
| Race/Ethnicity (Anglo/AA/Asian/Native Hawaiian or other Pacific Islander/other) (%) | 35/12/7/1/1 (63/21/12/2/2) | 24/9/5/1/0 (62/23/13/2/0) | 0.948 |
| Weight (kg) | 87.2 ± 1.7 | 83.4 ± 2.2 | 0.1674 |
| BMI (kg/m2) | 31.1 ± 0.4 | 30.7 ± 0.4 | 0.3842 |
| Tree Nut | Pretzel | |||||||
|---|---|---|---|---|---|---|---|---|
| BL | 4 | 12 | 24 | BL | 4 | 12 | 24 | |
| Triglyceride (mg/dL) | 88 ± 4.8 | 88 ± 4.2 | 94 ± 5.9 | 85 ± 4.8 | 84 ± 6.8 | 79 ± 5.0 | 84 ± 5.3 | 84 ± 5.8 |
| Total Chol (mg/dL) | 169 ± 6.0 | 168 ± 6.0 | 169 ± 6.2 | 163 ± 5.6 | 172 ± 8.5 | 169 ± 8.5 | 174 ± 7.1 | 169 ± 7.8 |
| HDL-Chol (mg/dL) | 33 ± 1.2 | 33 ± 1.2 | 34 ± 1.2 | 33 ± 1.1 | 31 ± 1.5 | 30 ± 1.5 | 32 ± 1.5 | 32 ± 1.8 |
| Diastolic BP (mmHG) | 78 ± 1.2 | 77 ± 1.4 | 76 ± 1.2 * | 77 ± 1.3 | 76 ± 1.1 | 76 ± 1.2 | 74 ± 1.4 * | 74 ± 1.5 |
| Systolic BP (mmHG) | 123 ± 1.6 | 121 ± 1.9 | 121 ± 1.8 | 121 ± 1.8 | 121 ± 2 | 120 ± 2.3 | 120 ± 2.3 | 118 ± 1.9 |
| Heart Rate (BPM) | 74 ± 1.6 | 71 ± 1.5 * | 71 ± 1.3 ** | 71 ± 1.3 * | 73 ± 1.6 | 71 ± 1.7 | 71 ± 1.7 | 73 ± 1.5 |
| Fatty | Tree Nut | Pretzel | ||||
|---|---|---|---|---|---|---|
| Acids # | 0 | 12 | 24 | 0 | 12 | 24 |
| Myristic | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.4 ± 0.5 |
| Palmitic | 22.1 ± 1.8 | 22.0 ± 1.8 | 21.8 ± 1.5 | 22.3 ± 1.9 | 22.6 ± 2.1 | 22.6 ± 2.2 |
| Stearic | 7.1 ± 0.6 | 7.0 ± 0.5 | 7.0 ± 0.7 | 6.9 ± 0.6 | 6.7 ± 0.7 * | 6.9 ± 0.7 |
| Oleic | 20.8 ± 2.6 | 21.6 ± 2.4 * | 21.7 ± 2.7 * | 21.2 ± 2.9 | 21.7 ± 2.7 * | 21.2 ± 2.8 |
| Linoleic | 35.6 ± 4.0 | 35.3 ± 4.0 | 35.2 ± 4.1 | 34.8 ± 3.8 | 34.3 ± 3.8 | 34.7 ± 4.1 |
| Linolenic | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.7 ± 0.2 * |
| Arachidonic | 8.8 ± 2.3 | 8.6 ± 2.3 | 8.6 ± 2.1 | 9.0 ± 2.6 | 8.8 ± 2.3 | 8.6 ± 2.3 |
| EPA | 0.7 ± 0.5 | 0.6 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.5 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| DHA | 1.7 ± 0.7 | 1.6 ± 0.6 | 1.7 ± 0.6 | 1.6 ± 0.6 | 1.7 ± 0.7 | 1.7 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, S.; Henning, S.M.; Qin, T.; Pan, Y.; Yang, J.; Huang, J.; Tseng, C.-H.; Heber, D.; Li, Z. Mixed Tree Nut Snacks Compared to Refined Carbohydrate Snacks Resulted in Weight Loss and Increased Satiety during Both Weight Loss and Weight Maintenance: A 24-Week Randomized Controlled Trial. Nutrients 2021, 13, 1512. https://doi.org/10.3390/nu13051512
Wang J, Wang S, Henning SM, Qin T, Pan Y, Yang J, Huang J, Tseng C-H, Heber D, Li Z. Mixed Tree Nut Snacks Compared to Refined Carbohydrate Snacks Resulted in Weight Loss and Increased Satiety during Both Weight Loss and Weight Maintenance: A 24-Week Randomized Controlled Trial. Nutrients. 2021; 13(5):1512. https://doi.org/10.3390/nu13051512
Chicago/Turabian StyleWang, Jing, Sijia Wang, Susanne M. Henning, Tianyu Qin, Yajing Pan, Jieping Yang, Jianjun Huang, Chi-Hong Tseng, David Heber, and Zhaoping Li. 2021. "Mixed Tree Nut Snacks Compared to Refined Carbohydrate Snacks Resulted in Weight Loss and Increased Satiety during Both Weight Loss and Weight Maintenance: A 24-Week Randomized Controlled Trial" Nutrients 13, no. 5: 1512. https://doi.org/10.3390/nu13051512
APA StyleWang, J., Wang, S., Henning, S. M., Qin, T., Pan, Y., Yang, J., Huang, J., Tseng, C.-H., Heber, D., & Li, Z. (2021). Mixed Tree Nut Snacks Compared to Refined Carbohydrate Snacks Resulted in Weight Loss and Increased Satiety during Both Weight Loss and Weight Maintenance: A 24-Week Randomized Controlled Trial. Nutrients, 13(5), 1512. https://doi.org/10.3390/nu13051512






